Rapamycin has been shown to extend lifespan and reduce symptoms in a broad range of diseases and, at the cellular level, is known to slow down the rate at which proteins are made. But the new Salk research, published in the journal eLife, suggests that rapamycin could also target the neural damage associated with Leigh syndrome, a rare genetic disease, and potentially other forms of neurodegeneration as well.
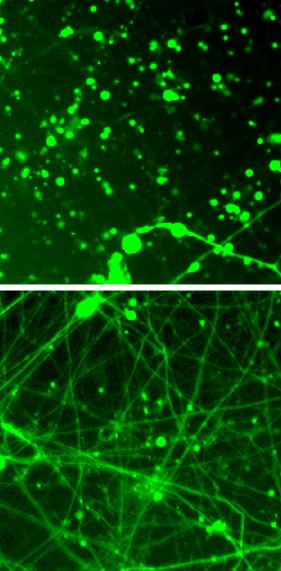
Salk scientists show how the drug rapamycin (bottom) rescues Leigh syndrome neurons.
Previous studies on rapamycin, which blocks a key energy sensor in cells, found that it can alter the immune system, extend lifespan and treat disorders, including lupus and Alzheimer’s disease. Researchers assumed that the drug prevented the neurodegeneration seen in Alzheimer’s by encouraging cells to degrade damaged components and aggregated proteins. But recent data hinted that the drug might also have an effect on the mitochondria, organelles that act as cells’ powerhouses, producing energy in the form of adenosine triphosphate (ATP).
Xinde Zheng, a research associate in the Hunter lab, was already studying the properties of cells affected by Leigh syndrome, whose inherited neurodegeneration is caused by a mutation in mitochondrial DNA that reduces ATP production. Zheng wondered how rapamycin would affect the neurons plagued by the diseased mitochondria. He and Hunter teamed up with the lab of Rusty Gage, a professor in Salk’s Laboratory of Genetics and holder of the Vi and John Adler Chair for Research on
Though cells must make proteins to survive, protein production is a highly
«Reducing protein production in aging neurons allows more energy for the cell to put toward folding proteins correctly and handling stress," says Zheng, the first author of the new paper. «The impact of our finding is that modulation of protein synthesis could be a general approach to treating neurodegeneration.»

From left: Xinde Zheng, Tony Hunter, Leah Boyer and Rusty Gage
«We are surprised and delighted that rapamycin’s effect to reduce protein synthesis as an
This is a good example of the value of studying a disease in a dish, according to Hunter. «It’s led to a lot of new insights into the underlying biology of this rare and understudied condition," he adds.
More work is needed to determine whether the findings on rapamycin hold true in animal models of Leigh syndrome and other neurodegenerative diseases, and to ascertain how exactly rapamycin is altering the metabolism of the cells.
Other researchers on the study were Mingji Jin, Youngsung Kim, Weiwei Fan, Cedric Bardy, Travis Berggren and Ronald M. Evans, all of the Salk Institute.
The work and the researchers involved were supported by grants from the National Institutes of Health, the Howard Hughes Medical Institute, a Calouste Gulbenkian Foundation Fellowship, a California Institute for Regenerative Medicine Postdoctoral Training Award, and the Helmsley Center for Genomic Medicine.
Source: http://www.salk.edu/news-release/tamping-down-neurons-energy-use-could-treat-neurodegeneration/

